Aim: Detailed molecular-level studies of well-defined planar Au, Pt, C and oxide surfaces in contact with various electrolytes, to elucidate the structure and dynamics of solvent and ions of the inner Stern part of the electric double layer, and to develop improved modeling approaches for the Stern layer.
Research
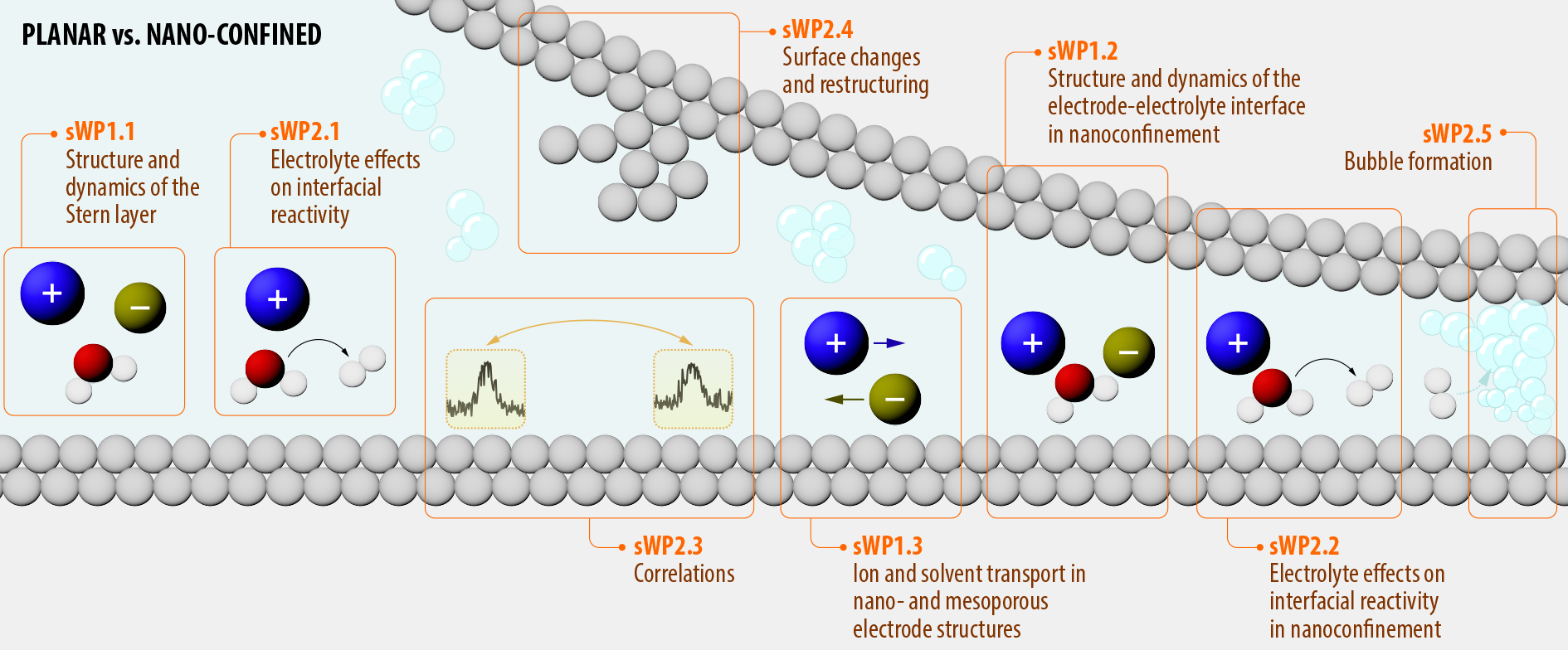
The research in ANION is divided into two Work Packages (WPs), WP1 and WP2, which are subdivided into three and six sub Work Packages (sWPs), respectively. A schematic overview of the WPs and their interrelation is given in the figure below. WP1 is uniquely devoted to the structure and dynamics of the electric double layer and the electrolyte in a planar and three-dimensional nanoscale environment. Environments and materials are chosen for their structural and chemical tunability and their ability to develop detailed computational models and model concepts. WP1 studies the nature of the still elusive so-called “inner” Stern layer on relevant electrode materials, how it changes in nanoconfinement, and elucidates the relevant transport modes by which charge moves in and out of these structures under charging and discharging. WP2 expands on WP1 by including reactivity in these well-defined and tuneable nanoscale environments, i.e. interfacial charge transfer, focusing on generic phenomena such as electrolyte effects, spatial and dynamic correlations, ion intercalation, electrocatalysis, and bubble formation. WP2 focuses specifically on relevant reactions such as lithium (and alkali metal) intercalation and discharge, hydrogen evolution, oxygen evolution, and CO2 reduction.
- Objectives
- Joint theory-experiment determination of the structure and properties of the solvent layers and the ion distribution (double-layer structure) near the surfaces of platinum, gold, C/graphene and oxide electrodes.
- New molecular models for the structure of the most inner part of the electric double layer, based on a multi-pronged electrochemical, spectroscopic and computational approach.
- Hypothesis
Under most practical electrolyte concentrations, the outer diffuse part of the electric double layer (Gouy- Chapman) near an electrode surface plays a less important role, and all reactive processes happen in the inner (Stern) part. The exact nature of the Stern layer is one of big unknowns in interfacial electrochemistry (and in colloid chemistry) as it is very sensitive to the nature of the electrode surface (chemical nature, geometry), the electrolyte components (solvent, ions), and the electrode potential (or surface charge).
Aim: To understand the impact of confinement of the electrolyte in pores (<10 nm) on the ion distribution and speciation throughout the pore, as a function of key parameters such as pore size and geometry, but also solid (pore wall) interfacial properties.
- Objectives
- Detailed information on and understanding of the impact of confinement of the electrolyte solution in pores (<10 nm) on the ion distribution and speciation
- New techniques (cryo-TEM) for studying electrolyte structures in nanoporous electrodes
- Hypothesis
Under nano-confinement, solvent molecules and ions assume a different (solvation) structure and a different distribution as a function of distance to the pore wall (electrode surface) than near a planar electrode surface. These differences depend on the length scale of the nanoconfinement (pore diameter) and the nature of the (chemical) interaction between the electrolyte constituents and the electrode material (pore wall). The interaction with the nanostructured electrode material has a direct effect, but nanoconfinement also induced indirect effects via a change in the structure and physical properties of the solvent. These changes influence the ion solubility, the ion mobility, and the charge storage capacity of the overall nanostructured electrode, and hence the efficiency with which reactions can be carried out at the electrode surface.
Aim: Investigate the charging dynamics of nanoconfined systems.
- Objectives
- Achieving detailed knowledge on the influence of nanoconfinement on diffusive, migrational, and advective mass and charge transport, notably due to the morphology of the porous structure, its chemical properties, wetting, and charge of the pore walls.
- Matching of state-of-the art theoretical modeling tools to selected experimental transport properties of nano- confined electrolytes.
- Hypothesis
Charge transport of ions in nanoporous materials is strongly influenced by surface effects including adsorption, surface conduction, streaming currents, diffusiophoresis, and inhomogeneities in transference numbers caused by electric double layer overlap that cause concentration polarization and rectification. The best strategy to disentangle these effects is to construct model systems with near-ideal geometry.
Aim: Reveal and understand the influence of the interfacial electrolyte structure and composition on HER, CO2RR and OER pathways and mechanism
- Objectives
- Detailed systematic insights into the effect of the local electrolyte structure (primarily cations) on the rates of HER and CO2RR
- Identification of cation-stabilized reaction pathways and corresponding active reaction intermediates by a combination of time-resolved heterodyne SFG, X-Ray Spectroscopy, and AIMD simulations.
- Hypothesis
The interfacial electrolyte, in particular the local cation identity and concentration, plays a key role in steering the reaction mechanism, both in terms of activity and selectivity, and can aid in the design of optimal reaction conditions.
Aim: Study, reveal and design the intricate interplay between electrolyte effects, mass transport and interfacial charge transfer in nanostructured electrodes for Li reduction, HER/HOR, OER and CO2RR
- Objectives
- Detailed systematic insights into the intricate interplay between electrolyte effects and nanostructure effects on the rates of OER, HER, Li (de)intercalation and CO2RR
- Design rules for the optimization of nanostructured electrodes materials for all four reactions, including kinetic simulation models incorporating surface kinetics and mass transport for the (semi-)quantitative prediction of activity and selectivity patterns
- Hypothesis
The central hypothesis of this Work Package is that significant activity and selectivity gains can be obtained by tuning the nanostructure of an electrode, and the corresponding electrolyte microenvironment, provided that we understand the above-mentioned intricate interplay. This implies strong collaborations between different sWPs, as well as a central role for modeling the connection between surface reactivity and mass transport in nanostructured architectures.
Aim: Investigation of correlations between activity at multiple reaction sites.
- Objectives
- Experimental nano- and surface science measurements (AMF, spectroscopy, amperometry) combined with theoretical modeling to provide a critical test of the hypothesis, namely that activity at one hot spot can influence that at another via a number of feedback mechanisms.
- Realization of a new nanofabricated device to detect such correlations directly.
- Identification of the various feedback mechanisms for HER and ORR reactivity.
- Hypothesis
Electrochemical conversion reactions do not take place uniformly over the electrode surface, but are centered at so-called hot spots. Apart from this spatially non-uniform activity, the activity at each hot spot also fluctuates in time. We postulate that several feedback mechanisms exist that can affect activity over a wide range of length scales, as well as time scales, leading to correlations in activity of different hot spots. Understanding such feedback mechanisms may prove essential for rational design of nanoscale electrodes.
Aim: Elucidation of restructuring of electrode surfaces during electrochemical reactions
- Objectives
- New methodology for imaging restructuring electrode surfaces, e.g. based environmental TEM (UU) and ONEM (UL)
- Understanding the instability and surface changes on cathodes during CO2RR
- Mechanistic insights in metal (de)intercalation processes in battery electrodes and dependence on battery chemistry and operating conditions
- Metal plating and electrode degradation mitigation procedures
- Hypothesis
Fundamental insight into the atomic and nanoscale origin of electrode degradation phenomena is crucial for prolonging the lifetime of electrochemical devices for electrochemical energy conversion. New imaging techniques in combination with state-of-the art operando spectroscopy and systematic experiments are essential in achieving these fundamental insights, which will allow improvement of [???].
Aim: Elucidation, understanding and control of electrochemical bubble formation in nanostructured electrodes
- Objectives
- Detailed insight and “design rules” in the nanoscale chemical and physical parameters that control bubble formation, bubble coalescence, and bubble release for a variety of relevant reactions.
- Development of protocols to minimize the deleterious effects of bubble evolution.
- Hypothesis
Tuning of electrode nanostructure in combination with electrolyte properties can optimize the current density of gas forming electrodes beyond purely catalytic activity.